Atipamezole Hydrochloride
Atipamezole, a synthetic drug with an imidazole structure, is a highly selective α2-adrenergic antagonist.1,2 Typically used to counteract the sedative, hypnotic, analgesic, and other effects produced by α2-adrenergic agonists (such as dexmedetomidine hydrochloride, medetomidine hydrochloride) or xylazine,3 atipamezole was approved by the FDA in 1996 to reverse the sedative and analgesic effects of medetomidine hydrochloride in dogs.4 It is currently approved to reverse the sedative and analgesic effects of medetomidine hydrochloride and dexmedetomidine hydrochloride (Dexdomitor, Pfizer).3
Atipamezole for veterinary use safely and reliably reverses the effects of these compounds and is widely used in small and large animal practices, as well as in wildlife applications, such as deer. Atipamezole reverses analgesic effects as well as sedative effects. Additional procedures for the control of pain may also be required.5
Pharmacology
Atipamezole is a central and peripheral α2-adrenergic antagonist.3 Adrenergic receptors are membrane-associated proteins through which catecholamines act to alter cell and organ functions.6 These can be divided into a and b types. α-Adrenergic receptors can be subdivided into subtypes (α1 and α2) according to their agonist and antagonist affinities. α2-Adrenergic receptors are located in the central nervous system, sympathetic nervous system, vasculature, and tissue (e.g., gastrointestinal tract, kidney, uterus).They exist presynaptically, postsynaptically, and extrasynaptically in the vasculature.3
α2-Adrenergic receptors are further divided into three pharmacologically different subtypes: α2A, α2B, and α2C. In the central nervous system, α2A is the prevalent subtype; the major noradrenergic nucleus in the brain stem, the locus coeruleus, contains only subtype α2A receptors.7 Several specific functions have been attributed to α2-adrenergic receptors. α2A Receptors mediate sedation, analgesia, hypotension, and bradycardia, while α2B receptors regulate peripheral vasoconstrictive effects. The α2C receptors mediate hypothermia. Mydriasis is attributable to activation of postsynaptic α2-adrenergic receptors.3
Atipamezole has a high affinity for all three α2-adrenergic receptor subtypes in both humans and rodents. It competitively displaces α2-adrenergic agonist drugs and rapidly blocks or reverses several drug effects. Atipamezole is more potent and selective than other α2-antagonists (e.g., yohimbine, tolazoline). Receptor binding studies in rodents have shown the α2-to-α1 selectivity ratio of atipamezole to be 8526 compared with 3240, 1620, 260, and 160 for dexmedetomidine, medetomidine, detomidine, and xylazine, respectively.5 The α2-to-α1 selectivity ratio for yohimbine, an indole alkaloid, is 40.
Atipamezole is highly selective: studies based on receptor binding and conducted using isolated organ preparations have shown that atipamezole has no affinity for or effects on other receptors (e.g., histaminergic, opiate, muscarinic, serotoninergic, dopaminergic, GABA-ergic, or benzodiazepine receptors). This selectivity minimizes the undesirable effects.3
Phamacokinetics
Atipamezole is a weak lipophilic base.1 Absorption after intramuscular administration is rapid; a peak plasma level is reached in about 10 minutes in dogs.5 Peak concentrations in tissue, including the brain, are two to three times higher than corresponding plasma levels. Atipamezole rapidly crosses the blood-brain barrier. The drug undergoes extensive hepatic biotransformation, and metabolites are primarily excreted in urine. The elimination half-life is 1.3 hours in rats and 2.6 hours in dogs.3
Indications
Atipamezole is used in the veterinary medicine as an effective agent to reverse the sedative/analgesic effects of the α2-adrenergic agonist drugs medetomidine, dexmedetomidine, romifidine, and xylazine.2,5 Atipamezole can also be used to shorten the duration of sedation, mobilize an animal after a noninvasive or minor surgical procedure, and reverse cardiovascular effects of the specific drugs listed above.3 Atipamezole produces a rapid reversal of medetomidine- or dexmedetomidine-induced bradycardia. This effect is of greater magnitude when dexmedetomidine is administered intravenously compared with intramuscular administration.6 If an animal has received combined anesthesia using ketamine, the veterinarian should wait at least 30 to 45 minutes after ketamine administration before administering atipamezole to reduce the risk of a challenging recovery.8
Atipamezole Dosage and Administration
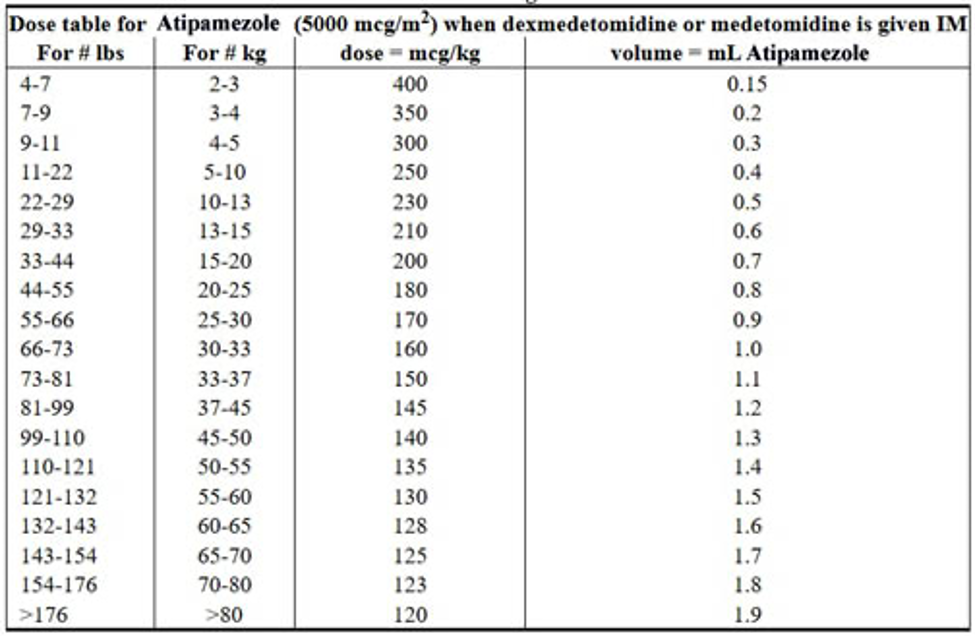
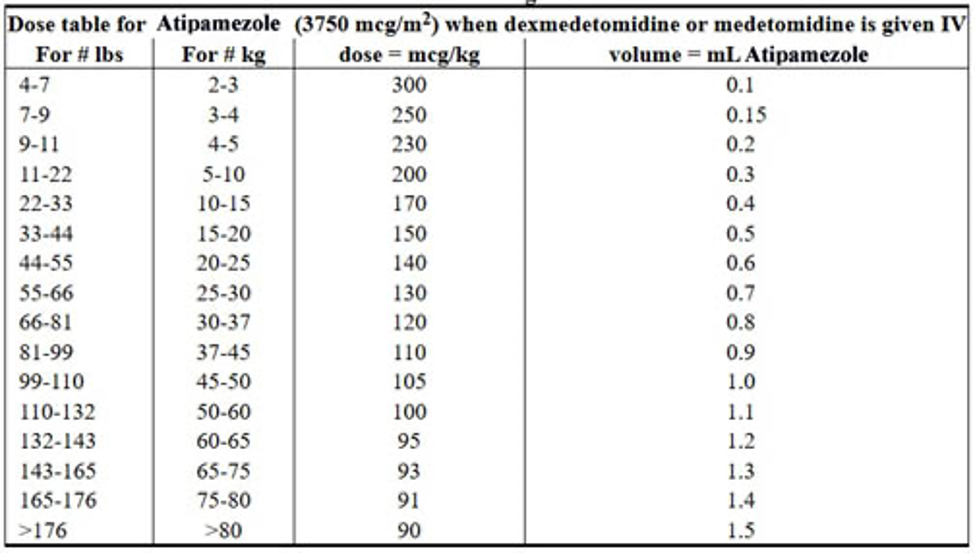
When administered IM at doses four to six times the medetomidine dose, atipamezole is highly effective in reversing medetomidine-induced sedation/analgesia, recumbency, and bradycardia in dogs within 3 to 7 minutes.8 Commercial preparations of atipamezole, medetomidine, and dexmedetomidine are usually formulated so that the concentration of atipamezole is five times the concentration of medetomidine and dexmedetomidine in the respective products. Therefore, the injection volume of the recommended dose of atipamezole is identical to the volume of the recommended dose of medetomidine or dexmedetomidine.5 Atipamezole dosages based on patient body weight are listed in the product prescribing information. If more than 30 minutes has elapsed since medetomidine administration, the dose of atipamezole should be reduced.3
Precautions
Atipamezole can produce an abrupt reversal of sedation and, presumably, analgesia induced by α2-agonist activity. Additional procedures for pain control may be required. The potential for apprehensive or aggressive behavior should be considered when handling dogs emerging from sedation, especially dogs predisposed to nervousness or fright.5 Staff members should use caution when handling dogs that have recently received atipamezole and should avoid situations in which the animal could fall.3
Direct exposure of the skin, eyes, or mouth to atipamezole may cause irritation; therefore, practitioners should avoid drug contact with eyes and skin and wear impervious gloves during drug administration.8 Atipamezole and anticholinergics can both cause dramatic increases in heart rate, so concurrent use of these drugs should be avoided.7
1The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th ed. Whitehouse Station, NJ: Merck & Co; 2001:148.
2Plumb DC. Atipamezole. Veterinary Drug Handbook. 4th ed. Ames, IA: Iowa State University Press; 2002:76-77.
4Andrade SF, Sakate M, Laposy CB, et al. Yohimbine and atipamezole on the treatment of experimentally induced amitraz intoxication in cats. Int J Appl Res Vet Med 2006;4(3):200-208.
5nih.gov.
6Biegon A, Mathis CA, Budinger TF. Quantitative in vitro and ex vivo autoradiography of the α2-adrenoceptor antagonist [3H] atipamezole. Eur J Pharmacol 1992;224(2):27-38.
7Lemke KA. Perioperative use of selective alpha-2 agonists and antagonists in small animals. Can Vet J 2004;45(6):475-480.
8Ko JCH, Fox SM, Mandsager RE. Sedative and cardiorespiratory effects of medetomidine, medetomidine-butorphanol, and medetomidine-ketamine in dogs. JAVMA 2000;216(10):1578-1583.
About NexGen Pharmaceuticals
NexGen Pharmaceuticals is an industry-leading veterinary compounding pharmacy, offering sterile and non-sterile compounding services nationwide. Unlike other veterinary compounding pharmacies, NexGen focuses on drugs that are difficult to find or are no longer available due to manufacturer discontinuance or have yet to be offered commercially for veterinary applications, but which still serve a critical need for our customers. We also specialize in wildlife pharmaceuticals, including sedatives and their antagonists, offering many unique options to serve a wide array of zoo animal and wildlife immobilization and anesthesia requirements.
Our pharmacists are also encouraged to develop strong working relationships with our veterinarians in order to better care for veterinary patients. Such relationships foster an ever-increasing knowledge base upon which pharmacists and veterinarians can draw, making both significantly more effective in their professional roles.
Disclaimer
The information contained in this blog post is general in nature and is intended for use as an informational aid. It does not cover all possible uses, actions, precautions, side effects, or interactions of the medications shown, nor is the information intended as medical advice or diagnosis for individual health problems or for making an evaluation as to the risks and benefits of using a particular medication. You should consult your veterinarian about diagnosis and treatment of any health problems. Information and statements have not been evaluated by the Food and Drug Administration ("FDA"), nor has the FDA approved the medications to diagnose, cure or prevent disease. Medications compounded by NexGen Pharmaceuticals are prepared at the direction of a veterinarian. NexGen Pharmaceuticals compounded veterinary preparations are not intended for use in food and food-producing animals.
NexGen Pharmaceuticals, LLC does not recommend, endorse or make any representation about the efficacy, appropriateness or suitability of any specific dosing, products, procedures, treatments, services, opinions, veterinary care providers or other information that may be contained in this blog post. NEXGEN PHARMACEUTICALS, LLC IS NOT RESPONSIBLE NOR LIABLE FOR ANY ADVICE, COURSE OF TREATMENT, DIAGNOSIS OR ANY OTHER INFORMATION, SERVICES OR PRODUCTS THAT YOU OBTAIN THROUGH THIS BLOG POST.



