Diclazuril for Horses: Preventing and Treating EPM

Equine Protozoal Myeloencephalitis (EPM) is a neurologic disease in horses caused by infection with the protozoan Sarcocystis neurona. The parasite infects horses when they ingest the S. neurona organism in contaminated feed or water. The definitive host of this organism is the opossum (Didelphis virginiana), which passes the organism in its feces. Horses are known as a “dead end” host for S. neurona, meaning that once infected, they are not a source of infection for other animals. Once the horse ingests S. neurona, the organism can penetrate the gastrointestinal tract, enter the bloodstream, and then enter the central nervous system.

EPM is endemic to the Americas, and was first described in the 1960s, although researchers were not yet aware of the causative agent. In the mid-1970s, it was determined that this disease was caused by a protozoan organism. The S. neurona organism was first isolated from the spinal cord of a horse with clinical signs of EPM in the early 1990s. In the late 1990s, another organism called Neospora hughesi was also shown to cause EPM in horses.1 This organism is widely distributed in the United States but, compared to S. neurona, is a relatively rare cause of EPM.
EPM: Clinical signs and Progression
EPM presents with a variety of neurologic signs, which can have a gradual onset, or be acute and severe. In general, horses with EPM present with asymmetric hindlimb paresis (weakness) and muscle atrophy. Occasionally, the first signs may be related to a cranial nerve deficit (blindness, facial nerve deficits) or a focal brain lesion (depression, seizures).2
Initial signs might include dysphagia, evidence of abnormal upper airway function, unusual or atypical lameness, or even seizures. Severely affected horses might have difficulty standing, walking, or swallowing and the disease can progress very rapidly. Early signs of EPM such as stumbling and frequent interference between limbs can be confused with lameness. Horses affected with EPM commonly exhibit a gradual progression in severity and range of clinical signs. In some cases, however, a gradual onset can give way to a sudden exacerbation in the severity of clinical illness, resulting in recumbency.
The vital signs in affected horses are usually normal and animals appear bright and alert. Some horses with EPM appear thin and mildly obtunded. Neurologic examination often reveals asymmetric ataxia, weakness, and spasticity involving all 4 limbs. Areas of hyporeflexia, hypalgesia, or complete sensory loss are occasionally present. The most common signs of brain/brainstem disease include obtundation, head tilt, facial nerve paralysis, and difficulty in swallowing, although signs are not necessarily limited to these areas.3
Without treatment, EPM will progress to severe paresis and possibly recumbency. This can occur over a matter of hours or years, depending on the case.1
EPM: Diagnosis and Treatment
A diagnosis of EPM is generally based on the clinical signs. These are highly variable, but most commonly, veterinarians suspect EPM when a horse presents with evidence of spinal cord disease. The veterinarian will then support a clinical diagnosis with serologic testing. Blood or cerebrospinal fluid (CSF) can be tested for antibodies to S. neurona. Considering that 45-65% of the horse population in North America has seropositivity for antibodies to S. neurona however, positive antibody tests are of limited use.
Cerebrospinal fluid (CSF) can also be tested for the S. neurona organism. CSF can be safely collected from the horse while it is standing, but sample contamination is more likely.1 The likelihood of contamination is reduced when CSF is collected with the horse under general anesthesia.
The history of EPM treatment is nearly as convoluted and frustrating (on the part of researchers and veterinarians) as was the quest to identify the pathogen responsible for EPM throughout the 1970s and 1980s. Prior to the approval of drugs like diclazuril, ponazuril and toltrazuril, veterinarians usually prescribed oral trimethoprim/sulfonamide antibiotics in combination with pyrimethamine for 12 to16 weeks.
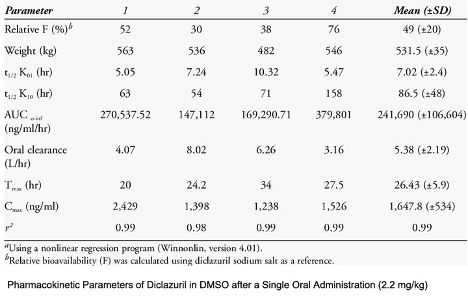
Diclazuril is available in suspension and injectable forms, and as an oral, alfalfa pellet-based medication that is typically prescribed for at least 28 days. Vitamin E supplementation is also frequently recommended due to its antioxidant properties. Horses with severe, acute neurologic deficits may also be treated initially with flunixin, phenylbutazone or DMSO.2
EPM Prevention
Since horses most commonly come into contact with S. neurona through the ingestion of feed or water containing opossum feces, prevention relies on reducing (or ideally, eliminating) the chances of opossum feces being present. Measures should be taken to dissuade opossums from venturing into horse feeding areas, and preventing them from accessing those areas if they do manage to enter a facility. Horse and pet feed should never be left out; open feed bags and trash should be kept in closed, secure containers.
Such items as discarded foodstuffs, fallen fruit and bird feeders are attractive to wildlife such as opossums, so barns and stables should be regularly policed for these items. “Outbuildings on the property, such as storage sheds that are rarely entered, can also serve as attractive hiding places for wildlife such as opossums. Making a point to enter these buildings regularly can help discourage opossums from taking up residence in these locations. Finally, opossums themselves can be trapped and relocated if the above measures fail to discourage them from frequenting a particular property.”4
1aaep.org.
2Dirikolu, Levent & Foreman, Jonathan & Tobin, Thomas. (2013). Current Therapeutic Approaches to Equine Protozoal Myeloencephalitis. Journal of the American Veterinary Medical Association. 242. 482-91. 10.2460/javma.242.4.482.
3Reed, S. M. et al. Equine Protozoal Myeloencephalitis: An Updated Consensus Statement with a Focus on Parasite Biology, Diagnosis, Treatment, and Prevention. Journal of veterinary internal medicine vol. 30, 2 (2016): 491-502. doi:10.1111/jvim.13834.
4Yang J., Ellison S., et. al. Immune response to Sarcocystis neurona infection in naturally infected horses with equine protozoal myeloencephalitis. J Parasitol. 3-4, 2006, Vol. 138, pp. 200-10.
About NexGen Pharmaceuticals
NexGen Pharmaceuticals is an industry-leading veterinary compounding pharmacy, offering sterile and non-sterile compounding services nationwide. Unlike other veterinary compounding pharmacies, NexGen focuses on drugs that are difficult to find or are no longer available due to manufacturer discontinuance or have yet to be offered commercially for veterinary applications, but which still serve a critical need for our customers. We also specialize in wildlife pharmaceuticals, including sedatives and their antagonists, offering many unique options to serve a wide array of zoo animal and wildlife immobilization and anesthesia requirements.
Our pharmacists are also encouraged to develop strong working relationships with our veterinarians in order to better care for veterinary patients. Such relationships foster an ever-increasing knowledge base upon which pharmacists and veterinarians can draw, making both significantly more effective in their professional roles.
Disclaimer
The information contained in this blog post is general in nature and is intended for use as an informational aid. It does not cover all possible uses, actions, precautions, side effects, or interactions of the medications shown, nor is the information intended as medical advice or diagnosis for individual health problems or for making an evaluation as to the risks and benefits of using a particular medication. You should consult your veterinarian about diagnosis and treatment of any health problems. Information and statements have not been evaluated by the Food and Drug Administration ("FDA"), nor has the FDA approved the medications to diagnose, cure or prevent disease. Medications compounded by NexGen Pharmaceuticals are prepared at the direction of a veterinarian. NexGen Pharmaceuticals compounded veterinary preparations are not intended for use in food and food-producing animals.
NexGen Pharmaceuticals, LLC does not recommend, endorse or make any representation about the efficacy, appropriateness or suitability of any specific dosing, products, procedures, treatments, services, opinions, veterinary care providers or other information that may be contained in this blog post. NEXGEN PHARMACEUTICALS, LLC IS NOT RESPONSIBLE NOR LIABLE FOR ANY ADVICE, COURSE OF TREATMENT, DIAGNOSIS OR ANY OTHER INFORMATION, SERVICES OR PRODUCTS THAT YOU OBTAIN THROUGH THIS BLOG POST.



